

Contact Us



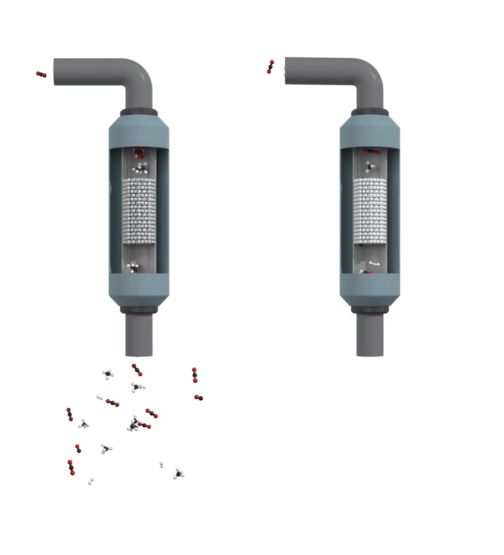
(∆H = +286 kJ/mol): Endothermic reaction
Contact
yllee@kmu.ac.kr
Department of Chemical Engineering, Keimyung University, Dalgubeoldaero 1095, Dalseo-Gu, Daegu, Republic of Korea
대구광역시 달서구 달구벌대로 1095 계명대학교 화학공학전공, 공과대학 3호관 3319호